MINI RESEARCH GRANTS
Application Due Date: February 19, 2021, 5:00pm
Download the Funding Opportunity Announcement
OVERVIEW
The primary objective of the Oklahoma IDeA Network of Biomedical Research Excellence (OK-INBRE) Collaborative Grant program is to foster research interactions between faculty at the OK-INBRE primarily undergraduate institutions so that faculty researchers may gain valuable experience in designing, conducting and reporting biomedical research, thus enhancing their ability to compete for extramural funding beyond the local level.
OK-INBRE expects to fund at least two Collaborative Grants. Funding is anticipated to be $25,000 in direct costs per award plus applicable F&A for OK-INBRE network institutions.
The proposed project must align with one of the biomedical research themes of the OK-INBRE program: Cancer, Developmental Biology or Infectious Diseases.
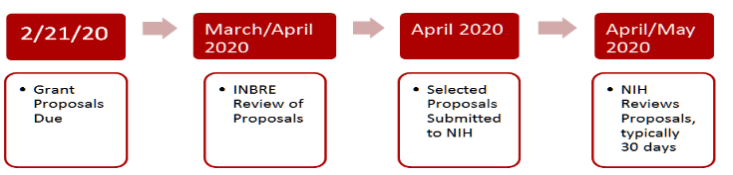
All selected projects must be approved by NIH before work on the project can begin.
Faculty may submit more than one application, provided each application is scientifically distinct. While faculty may apply for OK-INBRE Collaborative grants, Mini-Grants, and Research Project Investigator Awards simultaneously, only one award may be accepted.
Refer programmatic questions to Dawn Hammon at dawn-hammon@ouhsc.edu or 405.271.2133 x46613, or Darrin Akins at darrin-akins@ouhsc.edu.
PRINCIPAL INVESTIGATOR ELIGIBILITY
Investigators must meet the NIH definition of Early Stage Investigator (ESI) or New Investigator. Information regarding ESI and New Investigator policies can be found here: https://grants.nih.gov/policy/early-investigators/index.htm
Principal Investigators must hold a full-time faculty appointment at one of the eligible institutions listed below. Individuals with modified titles or those who lack independent status and who do not qualify to apply for an NIH peer-reviewed investigator-initiated research project grant are not eligible for OK-INBRE funding.
OK-INBRE can support non-tenure track faculty with a justification/letter of support from the Departmental Chair that the institution is providing resources, such as lab space, for the faculty member to successfully carry out the project. The reviewers for the proposals must be confident that the applicant is supported by the institution, is a stable faculty member (e.g. not temporarily at the institution), and the faculty member can successfully perform the project.
EFFORT OF THE PRINCIPAL INVESTIGATOR
Institutions must permit a reasonable time commitment to the research enterprise. While it is not required that salary support be requested, the maximum allowable salary support for the PI is $7,500 (salary and fringe included) to ensure there is ample resources in the budget for other categories to carry out the project as proposed (e.g., supplies, reagents, travel, and student/technical salaries).
Support cannot be requested for adjunct replacement costs. The budget must indicate that salary support is for the investigator, even if it is the equivalent cost of an adjunct replacement.
|
ELIGIBLE INSTITUTIONS
|
|
|
|
|
|
University of Central Oklahoma
|
|
|
East Central University
|
|
|
Northeastern State University
|
|
|
Northwestern Oklahoma State University
|
|
|
Southeastern State University
|
|
|
Southwestern Oklahoma State University
|
|
|
Cameron University
|
|
|
Langston University
|
|
|
Rogers State University
|
|
|
Oklahoma Panhandle State University
|
|
|
University of Science and Arts of Oklahoma
|
|
BUDGET, ALLOWABLE COSTS, AND PROJECT PERIOD
Direct costs are limited to $25,000 for the project period. Facility and Administrative costs (F&A) may be requested in addition to the $25,000 in direct costs; however, the following institutions will be required to waive F&A: East Central University, Rogers State University, University of Science and Arts of Oklahoma, Northwestern Oklahoma State University, Oklahoma Panhandle State University. Awards at these institutions will be funded by the Oklahoma State Regents for Higher Education.
Funds may be used for (but are not limited to):
- PI salary support not to exceed $7,500 (salary and fringe combined)
- Personnel salary and wages needed to carry out the project including students, technicians, etc.
- Supplies
- Equipment
- Travel to one professional meeting for the PI and students, not to exceed $2,000
- Other costs such as animals, animal housing, software and publication costs
The earliest potential start date for the project is May 1, 2020; however, the project period will not begin until NIH reviews and approves the project. This may delay start of the project. Grant funds cannot be applied to a period prior to the NIH project approval date. The end date is April 30, 2021.
APPLICATION
Applications should be prepared using Arial 11 point font, single spaced, with minimum 0.5 inch margins. Page limits are indicated below.
The PHS 398 grant form pages are located at: https://grants.nih.gov/grants/funding/phs398/phs398.html
- Face Page (Form Page 1)
Check that boxes 4 and 5 regarding animal and human subjects are marked appropriately
- Summary, Relevance, Project/Performance Sites, Senior/Key Personnel, Other Significant Contributors, and Human Embryonic Stem Cells (Form Page 2)
- Research Grant Table of Contents (Form Page 3)
- Detailed Budget for Initial Budget Period (Form Page 4)
- Resources Format Page
- Checklist Format Page
- Research Plan (there is no form for this section)
4 page maximum - Illustrations and figures will be counted against the page limitation
Specific Aims
Background & Significance
Innovation
Preliminary Data if available
Experimental Plan
- Bibliography and references cited (there is no form for this section)
- Resource Sharing Plan
This section includes Data Sharing Plan, when applicable, and Sharing Model Organisms. For more information on data sharing, please see the NIH website at /grants/ policy/data_sharing/.
- Select Agents
- Identify any select agents to be used in the proposed research. Select agents are hazardous biological agents and toxins that HHS or USDA have identified as having the potential to pose a severe threat to public health and safety, to animal and plant health, or to animal and plant products. CDC maintains a list of HHS and USDA Select Agents and Toxins.
- Plan for Authentication of Key biological and/or chemical resources to be used
- Letters of Support
- Non-tenure track faculty must provide a letter of support from the Departmental Chair indicating continuing support of the faculty member’s research career and laboratory space
- Letters from research collaborators are allowed
- Letters from proposed subcontractors are allowed
- Biographical Sketch
Biosketch format: https://grants.nih.gov/grants/forms/biosketch.htm
- Include a list of grant applications for the last three years, irrespective of funding outcome
Include PMCID numbers for NIH-funded publications
- If the proposal will involve human subjects, include the sections below. For help on the following topics, visit the NIH human subjects site.
Human and Animal Use Approvals are not required at the time of submission, but they must be in place before the project can be submitted to the NIH for approval and work on the project can begin.
Protection of Human Subjects
Inclusion of Women and Minorities
Target/planned enrollment table
Inclusion of Children
- If the proposal will involve vertebrate animals, include the sections below. For help on the following topics, visit the NIH vertebrate animals site.
Human and Animal Use Approvals are not required at the time of submission, but they must be in place before the project can be submitted to the NIH for approval and work on the project can begin.
Site of Animal Work
Description of Procedures
Justifications
Minimization of Pain and Distress
Method of Euthanasia
APPLICATION SUBMISSION
The deadline for submitting applications is 5:00 pm on February 19, 2021.
Submit a single .pdf file of the proposal in color (if color figures are included) to Dawn Hammon, OK-INBRE Program Manager, at dawn-hammon@ouhsc.edu. A paper application is not required.
Please be sure to route your applications through the appropriate grants administration office at your institution.
Refer programmatic questions to Dawn Hammon at dawn-hammon@ouhsc.edu or 05.271.2133 x46613 or Darrin Akins at darrin-akins@ouhsc.edu.
APPLICATION REVIEW
Each application will be assigned to two reviewers who will independently evaluate the scientific merits of the proposal. A panel of expert biomedical research scientists will provide input on each proposal and recommend whether or not an application should be considered for funding. The application will be ranked according to its scientific merit using the NIH scale of 10 to 90 with 10 being the theoretical perfect score. Upon completion of the peer review process, each applicant will be provided with the faculty peer review committee critiques.
The scientific merit review will be based on the following criteria:
1. Feasibility and scientific merit
2. Soundness of the approach and research design
3. Quality and appropriateness of data analyses
4. Qualifications and experience of the investigator
5. The role played by undergraduate/graduate students/postdocs/fellows in the proposed research. You may describe prior student involvement in your lab if appropriate.
6. Potential of the research to leverage into a national, state, or foundation application
7. Previous publication and grant submission productivity of the applicant
TERMS OF AWARD
All selected projects must be submitted to NIH for approval before funds can be dispersed and the project can begin. This may delay the start date of the project.
- For selected projects involving human subjects or vertebrate animals, all Institutional Review Board (IRB), Institutional Animal Care and Use Committee (IACUC) approvals must be secured before the project can be submitted to NIH for approval and work on the project can commence.
- Radiation Safety Committee and Institutional Biosafety Committee protocols must also be approved by relevant review committees prior to funding of awards.
- The Investigator will be required to present their project and progress to the External Advisory Committee once per year.
- The Investigator will be required to submit a project progress report each year. Instructions will be provided by OK-INBRE each year.